Abstract
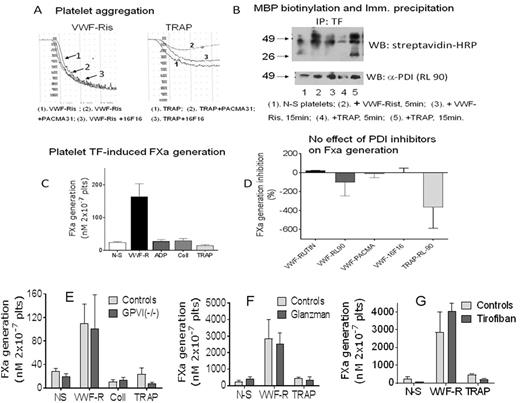
Megakaryocyte-synthesized Tissue Factor (TF) is transferred to platelets and carried "encrypted" in circulation. Platelet glycoprotein receptors, including GPIb and αIIbß3 integrin contain thiol groups representing redox sensitive sites. PDI is externalized and exposed on platelet membranes during the secretion process. Moreover, integrin ß subunits contain sequences with intrinsic PDI activity, as well as vicinal thiol groups which, in ß3 appear to regulate platelet activation. Several reports assign a mechanistic role to thiol isomerases in de-encrypting and activating TF in other cells, but the role of PDIs in platelet TF activation has not yet been explored. The extracellular TF domain proximal to the membrane contains Cys186-Cys209 disulfide, which is a likely target for PDIs. GPIb also has redox sensitive thiol groups. We have reported that VWF-induced GPIbα activation induces platelet TF activation (Atherosclerosis 2017;257:164). Now, we studied the role of PDI, GPVI and αIIbß3 in platelet TF activation. We tested polyclonal and RL-90 mAb α-PDI, as well as various PDI inhibitors (rutin, purified bacitracin, PACMA and 16F16), all of which inhibited aggregation and secretion of washed human platelets by TRAP, but not by VWF-Ristocetin (Fig A). The number of platelets binding Annexin V (flow cytometry) increased from ≈2-3% to >60% with both agonists. Basal platelet TF activity (24nM 2x10-7platelets) increased by 6-fold after stimulation with VWF-Ris, whereas ADP, TRAP and Collagen had no effect on FXa generation (Fig C). None of the PDI inhibitors had significant effect on FXa induced by VWF-Ris activation (Fig D). Membrane fractions obtained from thiol biotinylated platelets and IP with α-TF showed co-IP with PDI and TF thio-biotinylation, more pronounced after stimulation. This effect was not selective for VWF-Ris or TRAP.
VWF-Ristocetin stimulated platelets from 4 unrelated patients with homozygous GPVI deficiency (J Thromb Haemost 2013;11:1751) had normal generation of FXa (Fig E). Two patients with Glanzmann disease (Fig F) and 8 samples of washed platelets obtained from blood drawn with tirofiban added to the anticoagulant solution (Fig G) had very low FXa generation in resting platelets, which increased up to 100-fold after VWF-Ris stimulation. No FXa was induced by collagen or TRAP activation.
Conclusions. These observations strongly suggest that platelet PDI (and possibly PDI from other sources) is not involved in platelet TF activation and that PDI does not interfere with GPIbα function. Other mechanism(s) must be explored to explain the initiation of clotting by platelet TF. Moreover, our results highlight the specificity of GPIbα activation pathway in inducing physiological TF procoagulant activity, since neither GPVI nor αIIbß3 play a significant role in the clotting function of human platelets.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.